FOR CHILDREN AND YOUNG PEOPLE
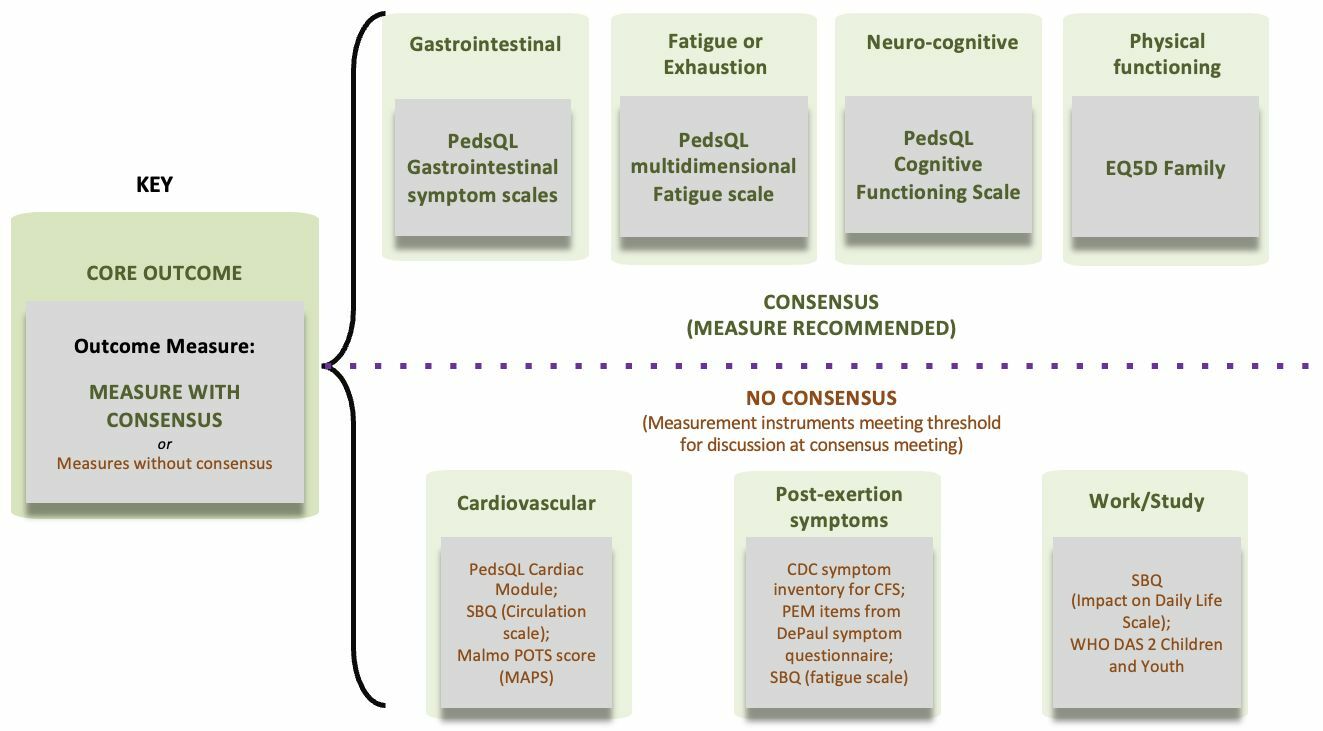
Through a consensus process, it was agreed that the following outcome measurement instruments should be employed: the 'PedsQL Multidimensional Fatigue Scale' for measuring 'Fatigue'; the 'PedsQL Gastrointestinal Symptom Scales' for evaluating 'Gastrointestinal' outcomes; the 'PedsQL Cognitive Functioning Scale' for assessing 'Neuro-cognitive' outcomes; and the 'EQ5D family' for 'Physical functioning' assessments.
Despite proposing outcome measurement instruments for the remaining three core outcomes -'cardiovascular', 'PEM', and 'work/occupational and study changes', consensus was not achieved. The number of measurement instruments for these outcomes was substantially reduced for and some suggestions were provided as a result of the study (please see figure).
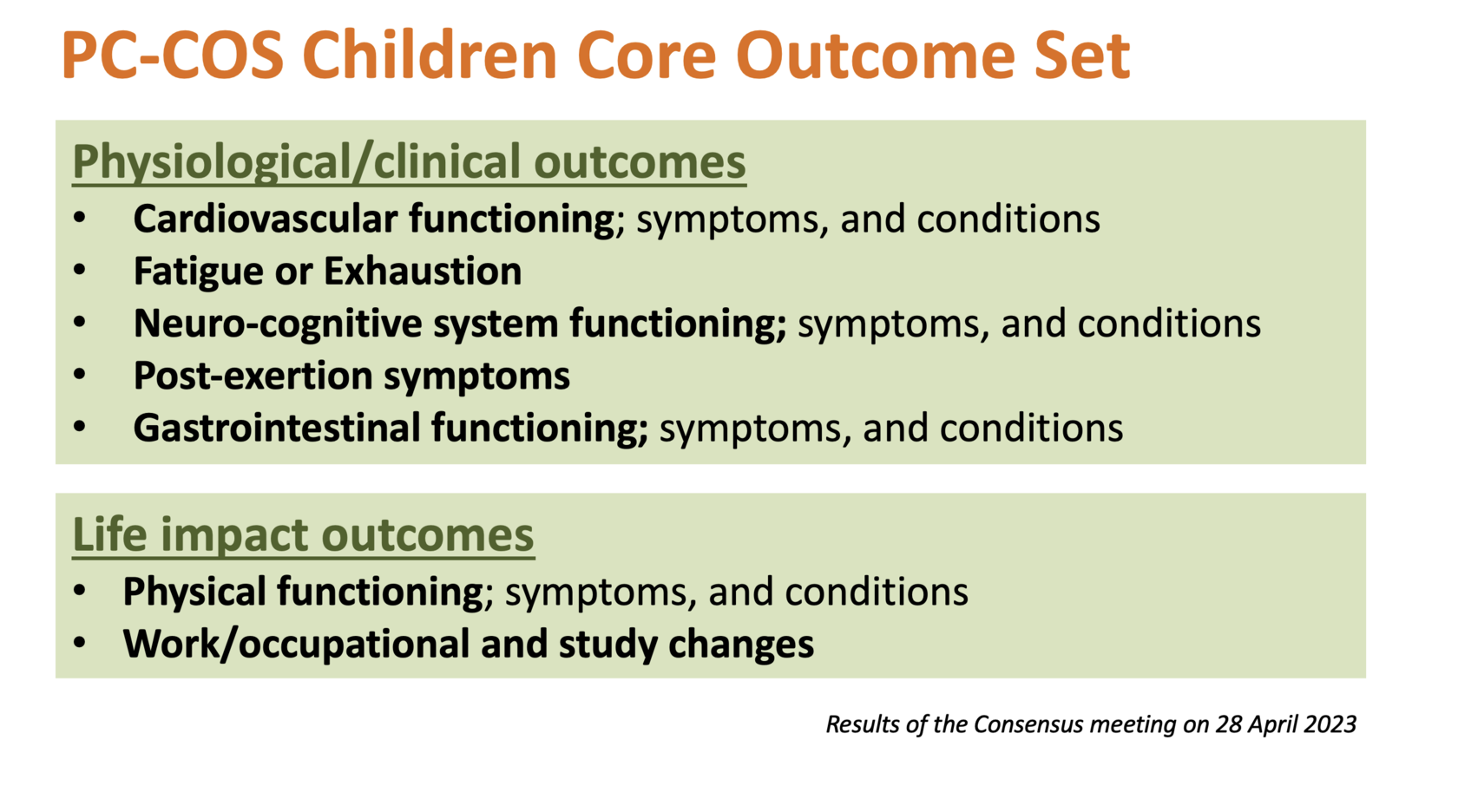
The PC-COS Children consensus meeting for the ‘what to measure’ stage has been held on 28 April 2023.
The full report of the consensus meeting will be available within a scientific manuscript of the study,
We wish to express our thanks to the participants of the study for their input and support of the project.


PC-COS Study Q&A on developing Post Covid-19 Condition Core Outcome Set in children and young people
Who is this study for?
- Children and young people (≤18 years old) who have experience of living with Long COVID
- Family and carers of children and young people (≤18 years old) with Long Covid
- Health professionals who have experience treating children and young people (≤18 years old) with Long Covid
- Researchers studying Long Covid in children and young people (≤18 years old)
- Organisations or people who use the research, for example those approving new treatments
- What is an outcome?When the health of an individual is evaluated, it is very important to know what effects of some condition are the most important and could affect their life the most. The same essential features inform the treatment testing and research.
In this project we want to find which features, or outcomes, are the most important in Long COVID in children and young people.
For example, in a study testing patients' recovery after hip fracture, an ‘outcome’ might include ‘mobility’ described as ‘changing basic body position; walking; moving around in different locations’. - Why are we doing this study?Currently there is no standardised approach for evaluating health and wellbeing of children and young people with Long COVID.
This problem is due, in part, to there being no universally agreed outcomes to measure in children and young people with Long COVID. Different researchers and health professionals around the world are measuring different outcomes. Hence, their results cannot be easily compared, potentially slowing the improvements in clinical care.
This study aims to find the outcomes that are essential to measure in research and clinical care. These outcomes are called core outcomes and will be included in a core outcome set. In the future, new studies and clinical care can ensure they measure these core outcomes so everyone works together more efficiently and potentially improve clinical care more quickly. - Does it matter how an outcome is measured?Please remember that we are interested in “what” outcomes should be measured. That means deciding which outcomes are the most meaningful for research or clinical care. Deciding how each outcome should be measured is important and will be decided in the next stage of the study.
- How will this study ensure children's and young people's involvement?This project is aiming to reach consensus on which outcomes should be measured in children and young people with Long COVID.
It is particularly important for children and young people to be involved in the decision-making process.- Children and young people (≤18 years old) with Long COVID.
- Carers of children and young people (≤18 years old) with Long COVID.
You can watch a short [3 minute] video explaining what core outcomes are, why they are important and how patients and health professionals are involved in developing them.
Subtitles are available in French, Portuguese, Dutch, Chinese, German, Spanish, Greek, Italian, Hungarian, Russian, Swedish, Bangla and Swahili languages.
This video has been created by the COMET Initiative team.
Review our Q/A re participation and confidentiality
You can find out more about each survey and future steps below.
- You can stop being part of the study at any time, without giving a reason, but we will keep information about you that we already have.
- We need to manage your records in specific ways for the research to be reliable. This means that we won’t be able to let you see or change the data we hold about you.
- If you agree to take part in this study, you will have the option to take part in future research using your data saved from this study.
Survey 1 (15-20 minutes to complete):
In the first survey, you will view a list of outcomes.
- For each outcome, you will rate how important you think it is to measure that outcome for all future research and clinical care of Long COVID.
- At the end of the survey, you can also suggest any other important outcomes are missing from the list.
- You can add comments to explain your decisions.
Survey 2 (15-20 minutes to complete):
Once the second survey is ready, you will see the same list of outcomes (apart from those reaching an a priori criteria for consensus) from survey 1 with a reminder of your own rating.
This time, you will also see a chart that shows how groups of people who took the first survey rated each outcome. This is a chance for you to consider the opinions of others and to reflect on your own previous ratings.
After these two surveys
After these two surveys there is the possibility of the need for an ‘Online Consensus Meeting’ to which a smaller subgroup of people with lived experience, health professionals, researchers and other groups representatives will be invited to discuss the results and agree the final core outcomes.
If you would like to be considered for a place at this meeting you can tell us at the end of the second survey.
Results of the Study
You will not be identified in the publication unless you tell us (at the end of the second survey) that you want to be acknowledged for your contribution. If this is the case, your name will be included in a specific acknowledgements section of the publication. We will not present your individual thoughts on outcomes in the publication; rather all results will be presented according to the different groups that took part (i.e. people with lived experience, health professionals, researchers, etc.).
Next Steps
After the above has been completed, a second stage will decide how to measure each of the core outcomes.
Taking part in the online surveys now does not mean that you must take part in future studies. However, when you register to take part, you can tell us if you would like to be kept up to date, by email, with opportunities to take part in the future.
We are an international team of health professionals, researchers and people with lived experience of Long COVID. Our goal is to improve research and clinical care by identifying the most important outcomes of Long COVID through collecting the views of different people from around the world.
In June 2022 our team successfully completed a similar study in adults. More than 1500 people of different backgrounds from more than 70 countries participated in the PC-COS project and reached consensus on core outcomes in post-COVID-19 condition in adults. The results were published in The Lancet Respiratory Medicine, a peer-reviewed scientific journal.
Please click here to view the paper.




























Chernyawskaya