Outcome measurement instruments
Post-COVID Condition/Long Covid Core Outcome Measurement Set (How to measure?)
The online consensus meeting for the ‘how to measure’ stage of the PC-COS study was held on September 29, 2022. This meeting was conducted after completion of a large international Delphi consensus process involving 594 stakeholders (people with Long COVID and their families, and health professionals/researchers with and without Long COVID). The process aimed to reach consensus, where possible, regarding recommended outcome measurement instruments for the core outcome domains identified in the preceding ‘what to measure’ stage of the PC-COS study.
Key results
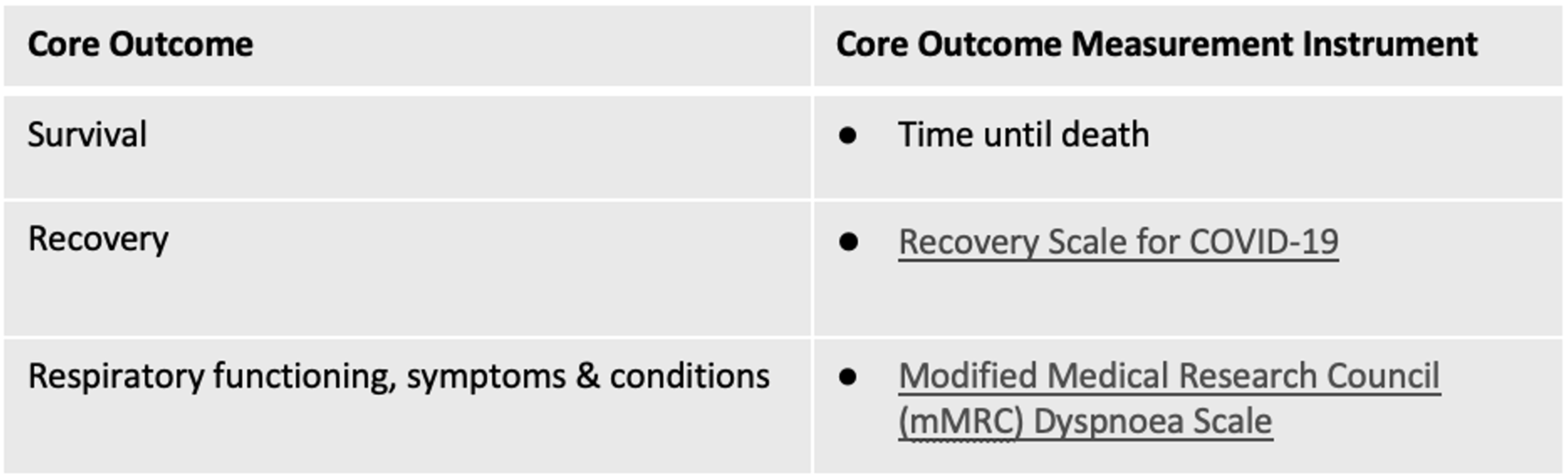
On the basis of this consensus process, in combination with results of a prior rigorous international consensus process for core outcome measures for acute COVID-19, three outcome measurement instruments are recommended for use in all studies and clinical practice in the field of Post COVID Condition/Long COVID (PCC/LC), also known as Post Acute Sequelae of COVID-19 (PASC):
On the basis of this consensus process, in combination with results of a prior rigorous international consensus process for core outcome measures for acute COVID-19, three outcome measurement instruments are recommended for use in all studies and clinical practice in the field of Post COVID Condition/Long COVID (PCC/LC), also known as Post Acute Sequelae of COVID-19 (PASC):
This Core Outcome Measurement Set (above) is the minimum set of measurement instruments recommended for all studies of people with PCC/LC. Specific studies, based on their objectives, would be expected to include additional study-specific measures, in addition to appropriate measurement instruments for all other PCC/LC Core Outcomes that did not achieve consensus for a recommended measurement instrument (see “Other Results” section for considerations).
Other results
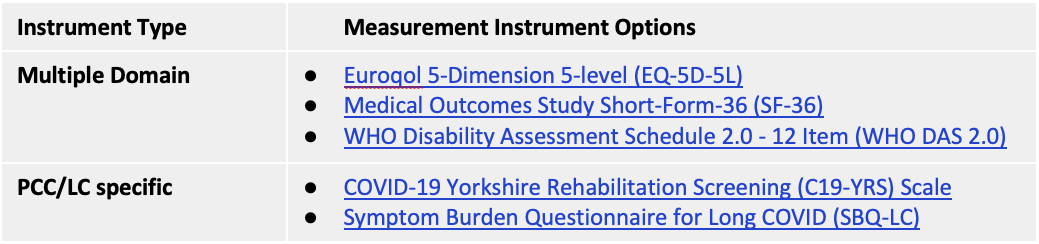
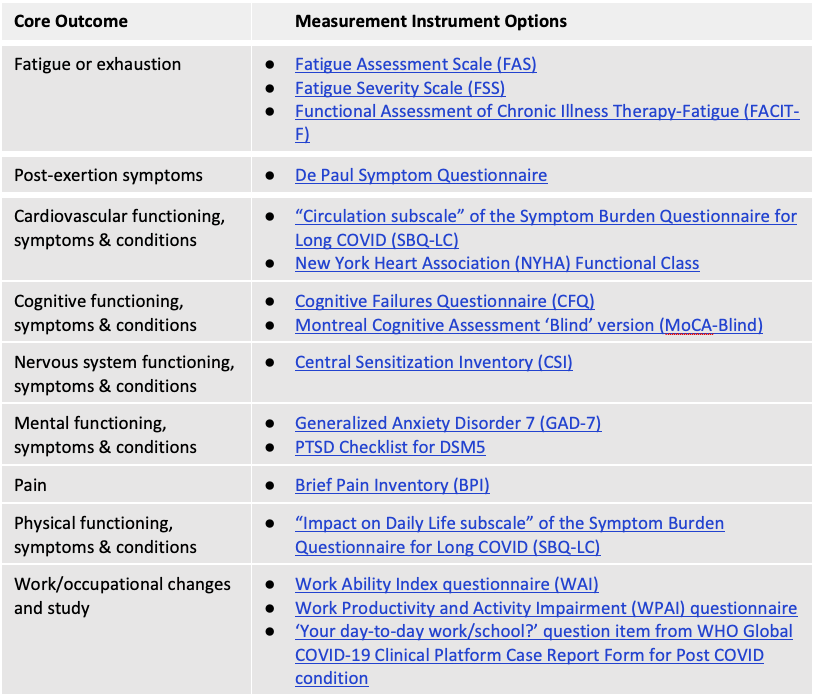
Consensus was not reached in recommending outcome measurement instruments for the remaining 9 core outcome domains in the PCC/LC Core Outcome Set. The Table, directly below, indicates measurement instruments with the greatest level of support based on the consensus process. Hyperlinks are to the ‘instrument cards’ used in the delphi study with summaries of key information and links to an example of the instrument. At least one of these measures can be considered for each of the Core Outcomes (i.e., where more than 1 measurement instrument is provided, selection of a single instrument may be appropriate to avoid redundancy and reduce respondent burden).
Consensus was not reached in recommending outcome measurement instruments for the remaining 9 core outcome domains in the PCC/LC Core Outcome Set. The Table, directly below, indicates measurement instruments with the greatest level of support based on the consensus process. Hyperlinks are to the ‘instrument cards’ used in the delphi study with summaries of key information and links to an example of the instrument. At least one of these measures can be considered for each of the Core Outcomes (i.e., where more than 1 measurement instrument is provided, selection of a single instrument may be appropriate to avoid redundancy and reduce respondent burden).
Consensus was also not reached for any multiple domain measurement instrument, nor any PCC/LC-specific measurement instrument. The below Table indicates measurement instruments with the highest level of support based on the consensus process. At the online consensus meeting, there was a very high level of support for future research focused on a consensus process regarding use of existing outcome measurement instruments versus Long COVID-specific instruments versus a combination of both types of instruments for PCC/LC research and clinical practice.
This study is funded by the UK National Institute for Health & Care Research (NIHR; Ref COV-LT2-00720).
The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health & Social Care.
The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health & Social Care.
Please click on the instrument to see the instrument card (link to PDF)
For Recovery Scale for COVID-19 please click on the instrument to see the article
For Recovery Scale for COVID-19 please click on the instrument to see the article
Please click on the instrument to see the instrument card (link to PDF)
Please click on the instrument to see the instrument card (link to PDF)