PC-COS Adults
I. Post-COVID Condition/Long Covid Core Outcome Set (What to measure?)
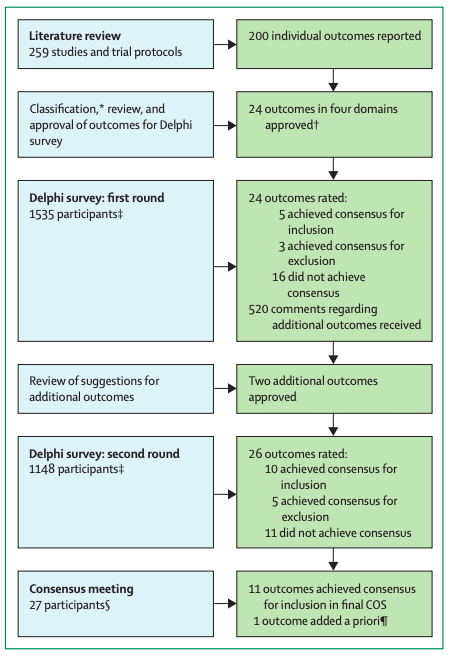

Overview of the COS development process
For the Delphi survey, all outcomes from the first round were included in the second round.
COMET=Core Outcome Measures in Effectiveness Trials.
COS=core outcome set.
*Outcomes were classified using COMET taxonomy.
†Outcomes were classified into survival, physiological or clinical, life impact, and resource use outcomes.
‡Participants were classified into three stakeholder groups: people with post-COVID-19 condition and family members or caregivers, health-care professionals and researchers with post-COVID-19 condition, and health-care professionals and researchers without post-COVID-19 condition.
§Participants were classified into two stakeholder groups: people with post-COVID-19 condition and family members or caregivers, and health-care professionals and researchers without post-COVID-19 condition.
¶Additional outcome was part of a previously published COS for COVID-19.
For the Delphi survey, all outcomes from the first round were included in the second round.
COMET=Core Outcome Measures in Effectiveness Trials.
COS=core outcome set.
*Outcomes were classified using COMET taxonomy.
†Outcomes were classified into survival, physiological or clinical, life impact, and resource use outcomes.
‡Participants were classified into three stakeholder groups: people with post-COVID-19 condition and family members or caregivers, health-care professionals and researchers with post-COVID-19 condition, and health-care professionals and researchers without post-COVID-19 condition.
§Participants were classified into two stakeholder groups: people with post-COVID-19 condition and family members or caregivers, and health-care professionals and researchers without post-COVID-19 condition.
¶Additional outcome was part of a previously published COS for COVID-19.
*Munblit, D., Nicholson, T., Akrami, A., Apfelbacher, C., Chen, J., De Groote, W., Diaz, J. V., Gorst, S. L., Harman, N., Kokorina, A., Olliaro, P., Parr, C., Preller, J., Schiess, N., Schmitt, J., Seylanova, N., Simpson, F., Tong, A., Needham, D. M., Williamson, P. R., … PC-COS project steering committee (2022). A core outcome set for post-COVID-19 condition in adults for use in clinical practice and research: an international Delphi consensus study. The Lancet. Respiratory medicine, 10(7), 715–724. https://doi.org/10.1016/S2213-2600(22)00169-2
A core outcome set for post-COVID-19 condition in adults for use in clinical practice and research: an international Delphi consensus study
Results are published
Results are published
Figure from the paper*
Figure from the paper*
II. Post-COVID Condition/Long Covid Core Outcome Measurement Set (How to measure?)
Summary of the consensus meeting results is available
Summary results of the Post COVID-19 Condition (Long COVID)
Core Outcome Set study’s ‘how to measure’ consensus process
The online consensus meeting for the ‘how to measure’ stage of the PC-COS study was held on September 29, 2022. This meeting was conducted after completion of a large international Delphi consensus process involving 594 stakeholders (people with Long COVID and their families, and health professionals/researchers with and without Long COVID). Twenty-five participants, from 14 countries, across 5 continents, attended the consensus meeting. The process aimed to reach consensus, where possible, regarding recommended outcome measurement instruments for the core outcome domains identified in the preceding ‘what to measure’ stage of the PC-COS study.
Core Outcome Set study’s ‘how to measure’ consensus process
The online consensus meeting for the ‘how to measure’ stage of the PC-COS study was held on September 29, 2022. This meeting was conducted after completion of a large international Delphi consensus process involving 594 stakeholders (people with Long COVID and their families, and health professionals/researchers with and without Long COVID). Twenty-five participants, from 14 countries, across 5 continents, attended the consensus meeting. The process aimed to reach consensus, where possible, regarding recommended outcome measurement instruments for the core outcome domains identified in the preceding ‘what to measure’ stage of the PC-COS study.
Key results
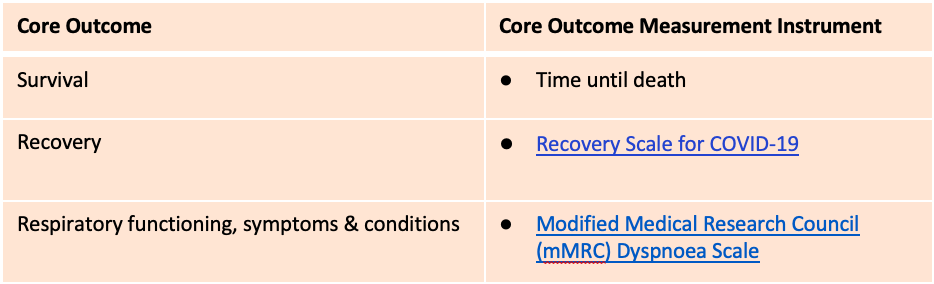
On the basis of this consensus process, in combination with results of a prior rigorous international consensus process for core outcome measures for acute COVID-19, three outcome measurement instruments are recommended for use in all studies and clinical practice in the field of Post COVID Condition/Long COVID (PCC/LC), also known as Post Acute Sequelae of COVID-19 (PASC):
On the basis of this consensus process, in combination with results of a prior rigorous international consensus process for core outcome measures for acute COVID-19, three outcome measurement instruments are recommended for use in all studies and clinical practice in the field of Post COVID Condition/Long COVID (PCC/LC), also known as Post Acute Sequelae of COVID-19 (PASC):
This Core Outcome Measurement Set (above) is the minimum set of measurement instruments recommended for all studies of people with PCC/LC. Specific studies, based on their objectives, would be expected to include additional study-specific measures, in addition to appropriate measurement instruments for all other PCC/LC Core Outcomes that did not achieve consensus for a recommended measurement instrument (see “Other Results” section for considerations).
Other results
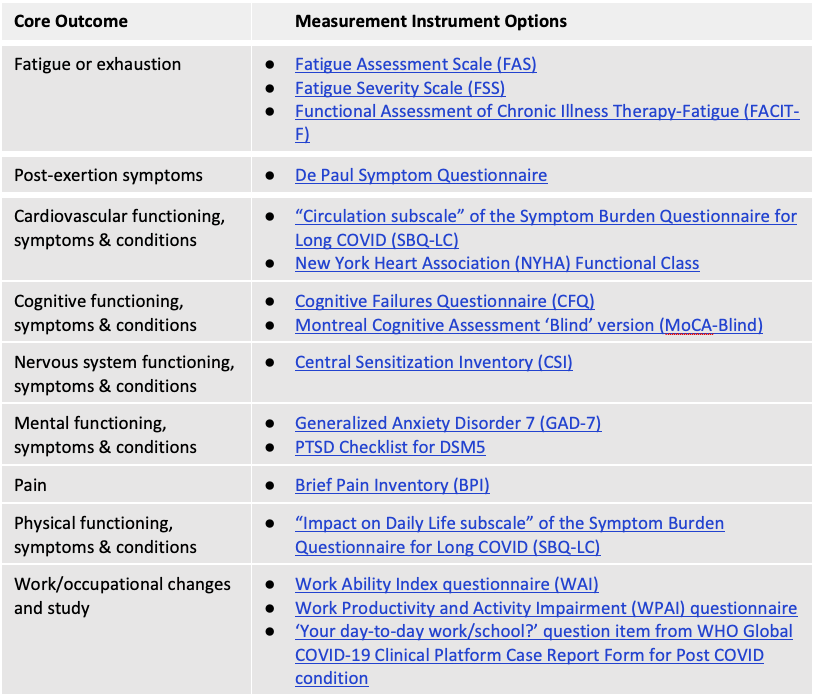
Consensus was not reached in recommending outcome measurement instruments for the remaining 9 core outcome domains in the PCC/LC Core Outcome Set. The Table, directly below, indicates measurement instruments with the greatest level of support based on the consensus process. Hyperlinks are to the ‘instrument cards’ used in the delphi study with summaries of key information and links to an example of the instrument. At least one of these measures can be considered for each of the Core Outcomes (i.e., where more than 1 measurement instrument is provided, selection of a single instrument may be appropriate to avoid redundancy and reduce respondent burden).
Consensus was not reached in recommending outcome measurement instruments for the remaining 9 core outcome domains in the PCC/LC Core Outcome Set. The Table, directly below, indicates measurement instruments with the greatest level of support based on the consensus process. Hyperlinks are to the ‘instrument cards’ used in the delphi study with summaries of key information and links to an example of the instrument. At least one of these measures can be considered for each of the Core Outcomes (i.e., where more than 1 measurement instrument is provided, selection of a single instrument may be appropriate to avoid redundancy and reduce respondent burden).
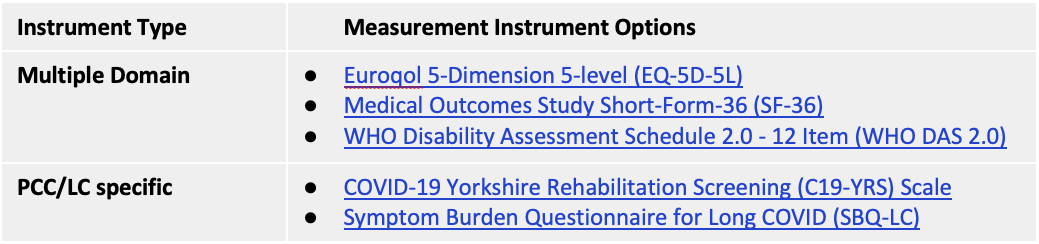
Consensus was also not reached for any multiple domain measurement instrument, nor any PCC/LC-specific measurement instrument. The below Table indicates measurement instruments with the highest level of support based on the consensus process. At the online consensus meeting, there was a very high level of support for future research focused on a consensus process regarding use of existing outcome measurement instruments versus Long COVID-specific instruments versus a combination of both types of instruments for PCC/LC research and clinical practice.
A report of the consensus meeting will be available shortly within a scientific manuscript of the study.
This study is funded by the UK National Institute for Health & Care Research (NIHR; Ref COV-LT2-00720). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health & Social Care.
10 October 2022
The PC-COS team
The PC-COS team
Time until death